Caustic Soda Flakes

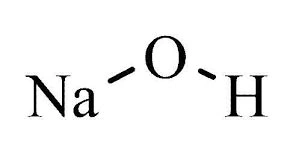
| Chemical Formula / Structure | NaOH | |
| Description | White flakes | |
| Specifications | Sodium Hydroxide (as NaOH) min Sodium Carbonate (as Na2CO3) max |
99.50% 0.40% |
| Reagents | Procedure | |
|---|---|---|
| 1 N HCl | Add 106 ml of 30% distilled/AR grade acid to 894 ml of distilled water. Mix well and keep the solution for some time. Mix again and standardise after 15 minutes Standardization: By means of weighing bottle transfer 1.8 to 2.2 gram of oven dried AR grade Na2CO3 in 250 ml clean glass beaker. Add 50ml of freshly boiled and cold distilled water and dissolve the material by means of glass rod. Titrate this against 1 N HCl to be standardised using ''A'' grade burette and 5 drops of methyle orange indicator Colour changes from yellow to light red. Note down the burette reading (BR) |
|
| 0.1 N. HCl | Pipette out 50ml of 1 N HCl into 500ml of columerised flask and dilute to 500ml | |
| Phenophthelein | Dissolve 0.1 gram of phenophthalein in 60 ml of ethyle alcohol. Make up the volume to 100ml with distilled water | |
| Methyle Orange | Dissolve 0.1 gram of methyle orange in 100ml of distilled water | |
| Applications | Used in a variety of industries such as | |
| * Pulp & paper | * Alumina | |
| * Textiles | * Soaps & Detergents | |
| * Dyes & Dyestuffs | * Pharmaceuticals | |
| * Water treatment | ||

